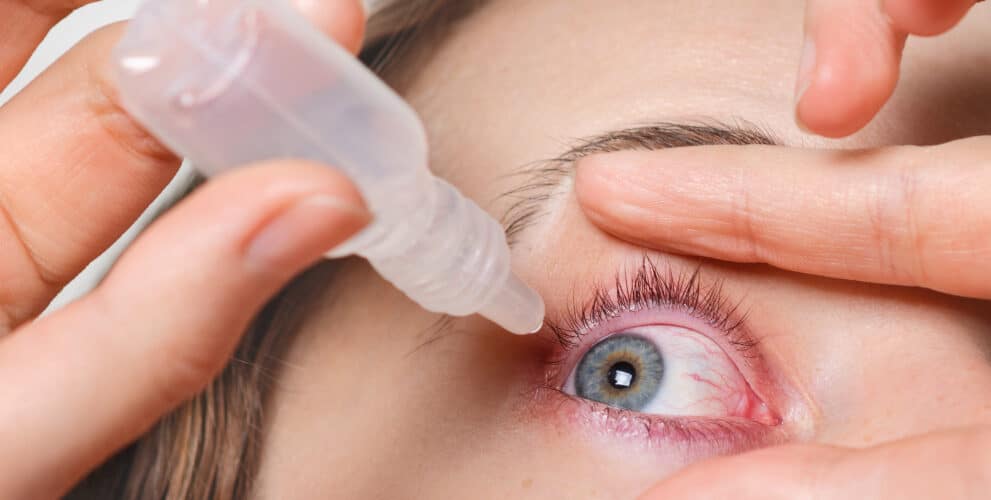
Ditch your glasses? FDA approves eye drops to fix your near sight
The once-daily drops are administered in two doses per eye, spaced two minutes apart
Author
Author
- admin / 6 months

- 0
- 3 min read
Author
The US Food and Drug Administration (FDA) has approved VIZZ™ (aceclidine ophthalmic solution 1.44%), a first-of-its-kind prescription eye drop for adults with presbyopia – the age-related condition that makes it difficult to focus on near objects.
According to the newly released prescribing information, “VIZZ is a cholinergic agonist indicated for the treatment of presbyopia in adults.”
The once-daily drops are administered in two doses per eye, spaced two minutes apart. The FDA notes that “contact lenses should be removed prior to the instillation of VIZZ, and may be reinserted 10 minutes after instillation.”
The Phase 3 trials showed dramatic results. In two randomized, double-masked studies involving 466 participants, “the proportion of participants gaining 3 lines or more in high contrast, distance-corrected near visual acuity at 40 cm… was statistically significantly greater in the VIZZ group compared to the control group at Day 1, Hour 3 — 65% vs. 12% in one trial, and 71% vs. 8% in the other (p<0.01).”
Some warnings
But the drug is not without warnings. The FDA prescribing document cautions that “patients may experience temporary dim or dark vision after instillation. Do not drive or operate machinery if vision is not clear… Exercise caution in night driving and other hazardous activities in poor illumination.”

It also highlights rare but serious risks: “Rare cases of retinal tear and detachment have been reported with miotics… Examination of the retina is advised in all patients prior to the initiation of treatment. Patients should be advised to seek immediate care with sudden onset of flashing lights, floaters, or vision loss.”
The most common side effects reported were “instillation site irritation (20%), dim vision (16%), and headache (13%).” Less frequent reactions included “conjunctival hyperemia (8%) and ocular hyperemia (7%).”
VIZZ is supplied in “sterile, single-dose vials” without preservatives and must be stored in refrigeration, though “it may be stored at room temperature up to 77°F (25°C) for 30 days.”
“Pinhole effect”
The drug works by producing a “pinhole effect” to increase depth of focus. “VIZZ is a predominately pupil-selective miotic that interacts with the iris with minimal ciliary muscle stimulation… resulting in a pinhole effect that extends depth of focus to improve vision,” the FDA document explains.
Patients are urged to follow handling instructions carefully: “Advise patients to avoid touching the tip of the single-dose vial to the eye or to any other surface… and to discard the opened single-dose vial and any remaining contents after use.”
Also read: Fact Check: Is using urine as an eye wash safe?
(Do you have a health-related claim that you would like us to fact-check? Send it to us, and we will fact-check it for you! You can send it on WhatsApp at +91-9311223141, mail us at hello@firstcheck.in, or click here to submit it online)